Abstract
Introduction. Novel treatment regimens that combine chemotherapy with targeted agents are being developed for DLBCL. Evaluation of these targeted agents in clinical trials may require prospective biomarker testing, such as immunohistochemistry (IHC), to select patients based on relevant molecular features. However, prospective biomarker testing takes days and there is concern that the delay in treatment may prevent enrollment of patients with the most aggressive disease, biasing the trial population. Here, using data from the Phase 3 GOYA trial (NCT01287741) in first-line DLBCL, we evaluate the impact on PFS of a treatment delay that simulates the additional screening time required for prospective IHC testing.
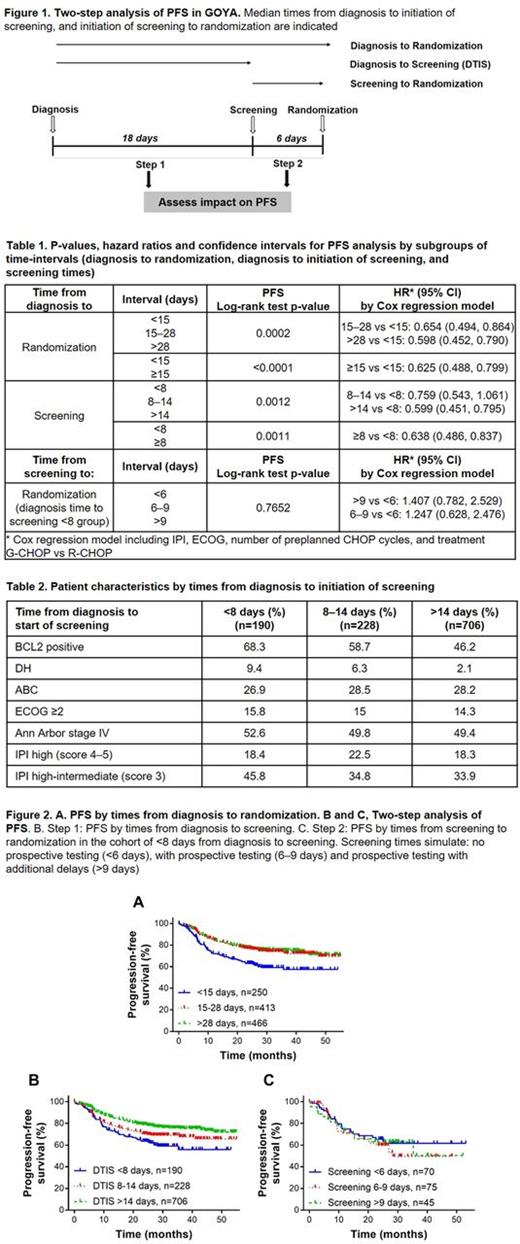
Methods. In GOYA, previously untreated DLBCL patients were randomized 1:1 to obinutuzumab or rituximab plus 6 or 8 cycles of CHOP. Median times from clinical diagnosis to initiation of screening ("DTIS interval"), and from initiation of screening to randomization ("screening time") were determined in patients with IPI 2-5 (n=1124 patients with timeline information available, no prospective testing). A two-step analysis of PFS was conducted by dividing the evaluable patient population into three groups of varying DTIS intervals (Figure 1, Step 1), and then into additional groups of varying screening times (Figure 1, Step 2). Groups for screening times were 1) <6 days (based on median screening time of 6 days in GOYA; 2) between 6-9 days (based on median screening time of 9 days in Roche clinical trial with prospective IHC testing - NCT02366143); and 3) above 9 days. We asked whether the clinical outcome (PFS), assessed by Kaplan-Meier survival analysis, correlated with either the DTIS interval (Step1) or the screening times (Step 2).
Results. Median time from diagnosis to randomization was 24 days (range 2 to 1106 days, 95th percentile of 66 days). Patients with times from diagnosis to randomization below 15 days (n=250) had significantly worse outcome than patients that were randomized in 15 or more days (n=413 for 15-28 days and n=466 for >28 days; p<0.0001; Table 1; Figure 2 A). 3-year PFS was 56% for patients with <15 days, 70% for 15-28 and 73% for >28 days from diagnosis to randomization. In Step 1 of the two-step analysis, DTIS times of <8 days, 8-14 days, and >14 days were observed for 190, 228 and 706 patients, respectively. Patients with a DTIS interval of <8 days were enriched with BCL2-positive and Double Hit status (Table 2). Patients with a DTIS interval of <8 days had shorter PFS than patients with longer DTIS intervals (p=0.0011 Table 1; Figure 2 B); 3-year PFS was 55% for patients with <8 days, 66% for patients with 8-14 days and 72% for patients with >14 days from diagnosis to screening; no additional difference in 3-year PFS was observed in subgroups beyond 14 days. In Step 2, we asked if a longer screening interval (screening to randomization in Figure 1), simulating additional time for prospective testing, affected patient outcome. Of the 190 patients with the shortest DTIS interval (<8 days from diagnosis to screening) and the shortest PFS from Step 1, 70 patients had screening times of <6 days, 75 between 6-9 days and 45 above 9 days (max 26 days). All cohorts showed indistinguishable PFS curves (p=0.7652; Table 1; Figure 2C). Similarly, no differences in PFS were observed for groups of increasing screening times in the DTIS cohorts of 8-14 days and >14 days (not shown).
Conclusion. In GOYA, short PFS was associated with <15 days from diagnosis to randomization and <8 days from diagnosis to screening, possibly attributable to high-risk biology, such as high expression of BCL2 and DH. Despite seemingly expedited work-up, these high-risk patients do poorly, highlighting the need for targeted therapies and innovative trial designs. In this example, additional screening time similar to the time required for prospective testing did not adversely affect PFS. Our results may have implications for designing precision medicine trials in DLBCL.
Szafer-Glusman: Genentech: Employment. Liu: F. Hoffmann-La Roche: Employment. Peale: Genentech / Roche: Employment, Equity Ownership. Farazi: Genentech: Employment. Ray: Genentech/Roche: Employment. Horn: F. Hoffmann-La Roche Ltd: Employment. Oestergaard: F. Hoffmann-La Roche Ltd: Employment. Kornacker: F. Hoffmann-La Roche: Employment; Roche: Equity Ownership. Sehn: Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria. Fingerle-Rowson: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Venstrom: Genentech, Inc.: Employment. Byrtek: Genentech: Employment, Equity Ownership. Punnoose: Genentech: Employment.
Author notes
Asterisk with author names denotes non-ASH members.